While watching this week's lecture on design control, I tried to figure out the main difference between Design Verification and Design Validation. Both are used to make sure that the design outputs align with the design inputs. They both must also be documented in the Design History File (DHF). Why are these two sections separate though because they seem to share similar purposes. Hopefully someone can clarify this with more details. Thank you!
This is a very common question in the medical device industry. The best way to look at it is to understand the definition of both Verification and Validation.
From 21 CFR 820:
- Design validation means establishing by objective evidence that device specifications conform with user needs and intended use(s)
- Verification means confirmation by examination and provision of objective evidence that specified requirements have been fulfilled.
In Simple words: Verification is to verify that you have made the device correctly according to your specification.
Validation is that you have made the device that the customer needs.
Design verification usually includes bench testing and lab testing to verify that your output meets your specifications.
Design Validation usually includes clinical studies and Human factor testing to verify that you made the device that the user needs.
Hope that helps,
Fady
To add to the previous response, design verifications and design validations are very similar however, design verifications verify the design inputs and outputs. Design validations check that the user required specifications are met, and the that the medical device is validated for its intended use. Design verifications occur through in house testing by engineers within the company, and validations are performed by nurses or doctors who would actually use the device. Validations are a good way to see how the user interacts with the device which gives a different perspective because the engineers/developers in house are so used to using the device during its development that they may miss some obvious things to correct with the device.
As per my understanding, Validation is concerned with making sure that the system meets the customer’s actual needs, while verification is concerned with whether the system is well-engineered, error-free, and so on. Verification will help to determine whether the software is of high quality, but it will not ensure that the system is useful at customer’s end.
To make it clearer, Verification can be seen like theoretical step. During making of any device, there must be design inputs and resulting outputs. To verify such, all the verification activities that are performed must meet these conditions.Whereas, Validation is performed to make sure that all user needs are met and that the device can meet its’ intended use. Validation is a more specialized and specific. This process is more technical and it involves all kinds of tests to make sure all requirements are met that were set forth by the design inputs.
-Hetal
In terms of verification it can be a considered a form of quality control in which processes, services or documents conform to specified requirements or regulations from the vendor, which may also be attributed within Installation qualification, which ensures that equipment that is installed meets all specifications, and is installed correctly, this can be considered to be a day to day task, while validation is looked upon as a method for which the device or system operates per the users intended purpose or in other words " Did I build what I need."
chris
There are major differences between design verification and design validation. For one thing, validation always occurs after verification, and in some case may not be required at all. Verification tests the specifications of the device separately, while the validation stage tests the device as a whole to see if it meets user needs. Each design input, and therefore design spec, is tested during verification until the device passes. It is isn't until this stage is over that the device goes through validation.
According to FDA,
820.30(f) Design verification shall confirm that the design outputs meets the design inputs requirements.
820.30(g) Design validation shall be performed under defined operating conditions on initial production units, lots or batches, or their equivalents. Design validation shall ensure that devices conform to intended uses, including the needs of the user and patient, and shall include testing of production units under actual or simulated use conditions. Design validation shall include software validation and risk analysis, where appropriate.
Addition to all above,
Design verification is all of the inspection, measurement, analysis, or testing that proves that one has the correct output specifications relative to the design input requirements. Design verification is less about testing and more about ensuring that as one moves from the initial design input requirements and subsequently translate them to more refined requirements.
Design validation, however, proves that with the final design output specifications, the design (finished, packaged, and labeled device) will perform as intended and as users need.
-Yiming
Like most of you mentioned before Validation and Verification are similar but the are not the same. In the verification process you are asking yourself "Did I make this product correctly". This means you are matching if the product meets the requirements and specifications and functions as necessary. Whereas in the validation process you are asking yourself "Did I make the correct product?". This means the product meets the claims it is being marketed for, the product meets the intended user needs, and the product is safe and effective. I use these two questions and this photo below to help me remember their differences.
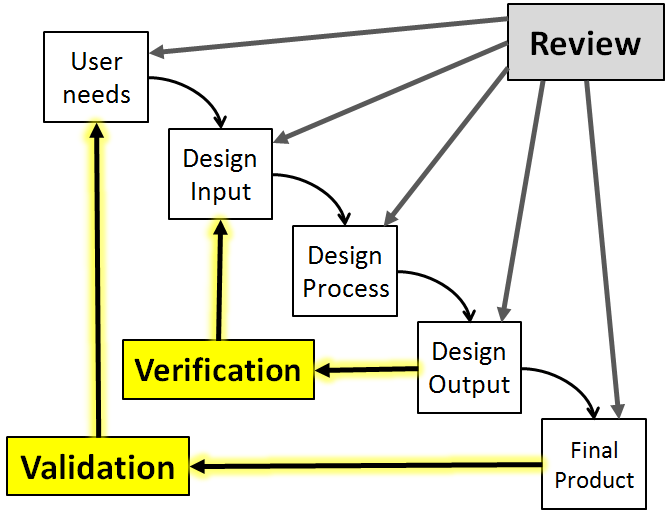
Design Verification & Validation seems to be interchangeable sometimes and can be very confusing. In medical device there is a way to separate the two. This is how I tell the difference between V&V. First, Design Verification is when a team correctly designs the device. While design validation, did the team make the correct design. Furthermore Design Verification seems to be more about testing and making sure the device is fully capable of accomplishing all FDA regulations. Design Verification and Validation are extremely need in the medical device industry. V&V are used to basically to make sure that the device achieves all requirements.
Great discussion so far!
Verification and validation are two critical elements of product development. One informs the elements of a product’s design, while the other looks at those design elements in the hands of users. Although often used synonymously, these alliterative terms sit on opposite sides of the product development life cycle.
Design verification ‘means confirming by examination and provision of objective evidence that specified requirements have been fulfilled
In contrast, design validation ‘means establishing by objective evidence that device specifications conform to user needs and intended use
this goes to validation vs verfiiction
so from the regulatroy refrence site we have the following,
"Validation is the process of checking whether the specification captures the customer's needs, while verification is the process of checking that the software meets the specification"
they are coupled in realtions just like QA VS QC but are different in nature.
here is an image of the graph showing the major diffrences
I think the following is a great way to think of verification vs validation:
Validation: Are we building the right system?
Verification: Are we building the system right?
In other words, validation is concerned with checking that the system will meet the customer’s actual needs, while verification is concerned with whether the system is well-engineered, error-free, and so on. Verification will help to determine whether the software is of high quality, but it will not ensure that the system is useful. The distinction between the two terms is largely to do with the role of specifications. Validation is the process of checking whether the specification captures the customer’s needs, while verification is the process of checking that the software meets the specification. Verification includes all the activities associated with the producing high quality software: testing, inspection, design analysis, specification analysis, and so on. It is a relatively objective process, in that if the various products and documents are expressed precisely enough, no subjective judgements should be needed in order to verify software. In contrast, validation is an extremely subjective process. It involves making subjective assessments of how well the (proposed) system addresses a real-world need. Validation includes activities such as requirements modeling, prototyping and user evaluation.
Validation and verification are very similar.
Verification is performed to check if the design follows to specifications by verifying the documents and the design. Verification uses different methods such as reviews and inspections. In this case the person checks the documents and the files. It can catch errors that cannot be found by doing the validation.
Validation is a testing to actual product. Validation is computer based execution. It’s done to check if the design meets the customers’ requirements and specifications. In most cases it is done after the validation is completed.
Thanks,
Grzegorz Galka
The verification refers to the process that deals with evaluating the customer needs and "verifying" that what the scope is focused in on is based on the customer needs. Validation refers to the process of complying with regulations and specifications based on the process of verification. It seems that the verification precedes the validation because like I've mentioned in multiple times, the scope of the project, which ultimate comes down to the customer needs as stated in the verification is the most important aspect of the project.
Validation and verification are frequently mentioned in tandem. They're often called V&V.
To get a good understanding of this, I wanted to add a few points that would explain the difference a little better.The literal meaning of validation means “the process of checking that something satisfies a certain criterion,”and verification can mean: “additional proof that something that was believed is correct”
A key distinction between design verification and design validation activities is that verification only requires that a single unit be assessed. What constitutes that single unit will vary depending on the intent of the verification. It might be one batch of raw material (for material performance tests), one machined part (for first article inspection), on one package sample (for integrity tests). The intent of verification is to confirm that the design outputs (i.e., the materials or components specified in design documents) meet the design input requirements.
Validations, on the other hand, require that studies be conducted to ensure that the device can be manufactured to meet design specifications on a consistent basis (i.e., process validation), and to ensure that the finished device is safe and effective for its intended purpose (i.e., design validation). For the process validation, multiple devices must be manufactured and evaluated to confirm that the production process is capable of producing devices within specifications on a consistent basis. For the design validation, multiple devices must be used to treat multiple patients to confirm that the treatment is safe and effectively.
